
Does Calcium (Ca) Form a Cation or Anion? YouTube
A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion. The metal cation is named first, followed by the nonmetal anion as illustrated in Figure 5.7.1 5.7. 1 for the compound BaCl 2. The word ion is dropped from both parts. Figure 5.7.1 5.7. 1: Naming BaCl2 B a C l 2.
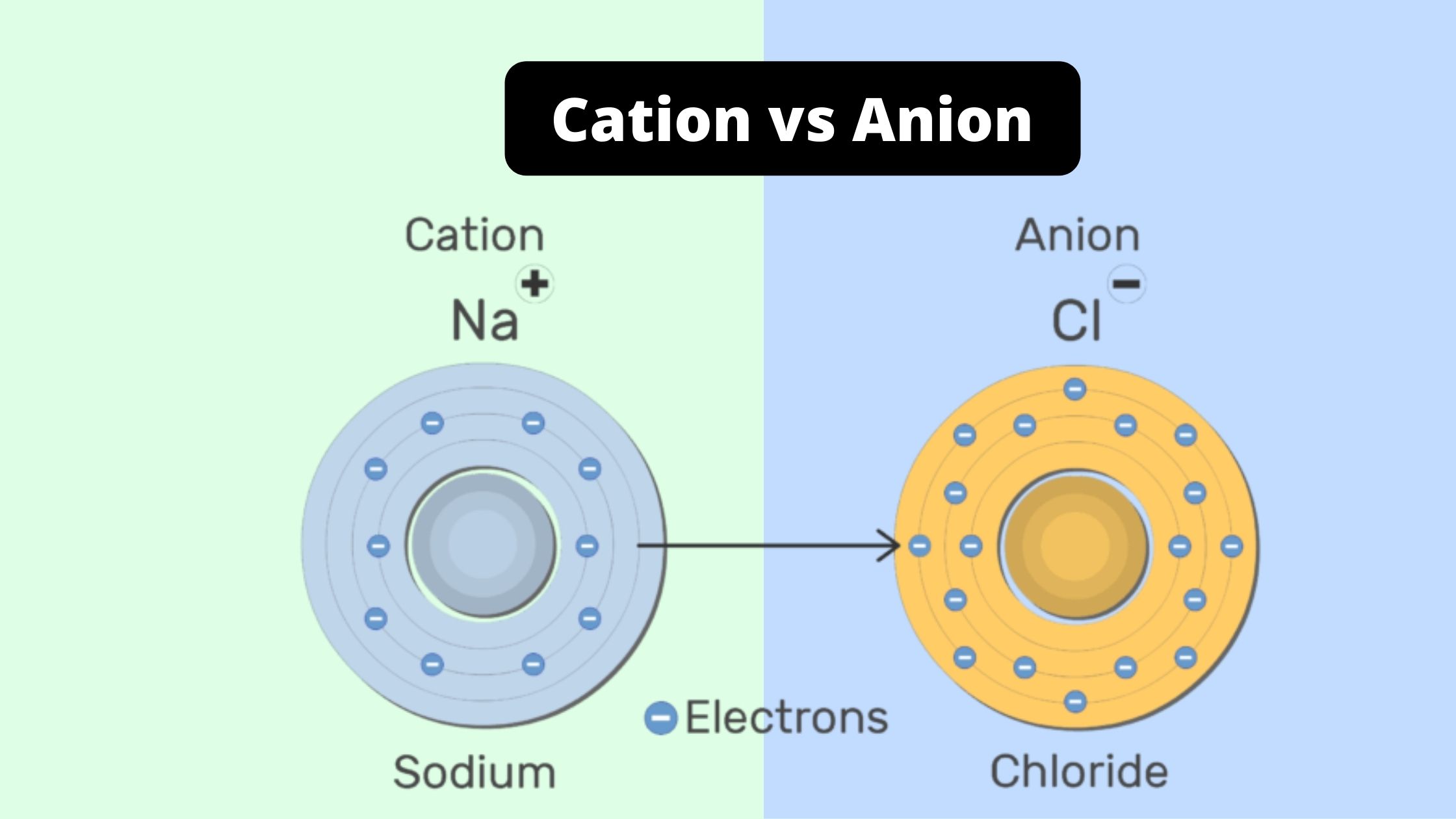
Difference Between Cation and Anion Cation vs Anion
Cation vs anion periodic table. It can be possible to predict whether an atom will form a cation or an anion based on its position on the periodic table. Halogens always form anions, alkali metals and alkaline earth metals always form cations. Most other metals form cations (e.g. iron, silver, nickel), whilst most other nonmetals typically form.

Thermal denaturation of Yfh1 at comparable anion and cation... Download Scientific Diagram
Exercise 8.11. 1. Write the complete ionic equation for. CaCl 2 ( aq) + Pb ( NO 3) 2 ( aq) → Ca ( NO 3) 2 ( aq) + PbCl 2 ( s) Answer. You may notice that in a complete ionic equation, some ions do not change their chemical form; they stay exactly the same on the reactant and product sides of the equation.

Binary ionic compound practice Name Perio... Physical Chemistry
Ionic bonding is the complete transfer of valence electron (s) between atoms. It is a type of chemical bond that generates two oppositely charged ions. In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion.
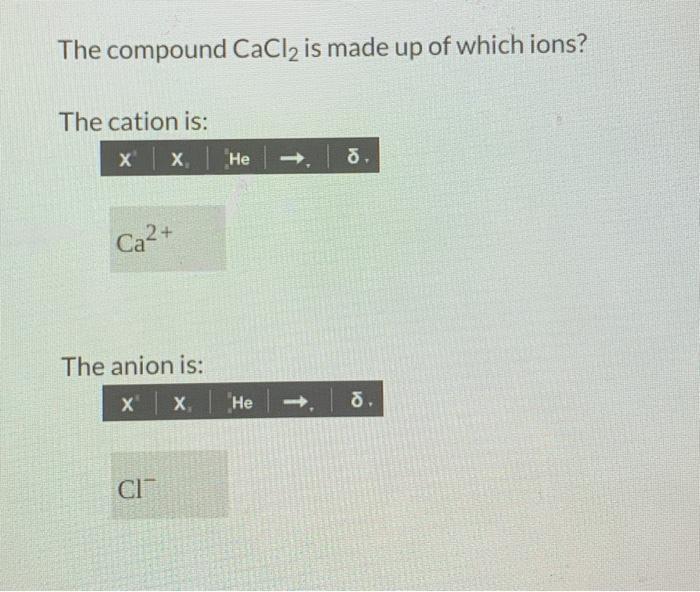
Solved The compound CaCl2 is made up of which ions? The
Common Ion Effect on Solubility. Consider, for example, the effect of adding a soluble salt, such as CaCl 2, to a saturated solution of calcium phosphate [Ca 3 (PO 4) 2 ]. We have seen that the solubility of Ca 3 (PO 4) 2 in water at 25°C is 1.14 × 10 −7 M ( Ksp = 2.07 × 10 −33 ).
SOLVED In the following ionic compound, Calcium chloride (CaCl2), how many electrons must
The formula of an ionic compound represents the lowest whole number ratio of cations to anions, it is as simple as that. Most cations have charges of [+1] through [+6] while most ions have charges of [-1] through [-3]. Trick: Set # of Anions = Charge of Cation and. set # of Cations = Charge of Anion.

Cation And Anion
The metadynamics AIMD simulations29 were performed to explore the free energy surface (FES) as a function of the coordination environment of Ca2+ ion in the CaCl2 aqueous solution. 1 CaCl2 and 64 water molecules were randomly packed in a cubic simulation box with a side length of

1 Show the formation of cacl2 by the transfer of electrons 2 identify the cation and anion
Solution Verified by Toppr CaCl2 is made up of two ions, The cation is Ca2+ and the anion is Cl− Was this answer helpful? 0 Similar Questions Q 1 Which of the following sets contain only isoelectronic ions? (i) Zn2+,Ca2+,Ga3+,Al3+ (ii) K+,Ca2+,Sc3+,Cl− (iii) P 3−,S2−,Cl−,K+ (iv) T i4+,Ar,Cr3+,v5+ View Solution Q 2

Plot of calcium chlorideextractable P (CaCl2P) against anion storage... Download Scientific
Precipitation Reactions is shared under a CC BY license and was authored, remixed, and/or curated by LibreTexts. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Whether or not such a reaction occurs can be determined by..
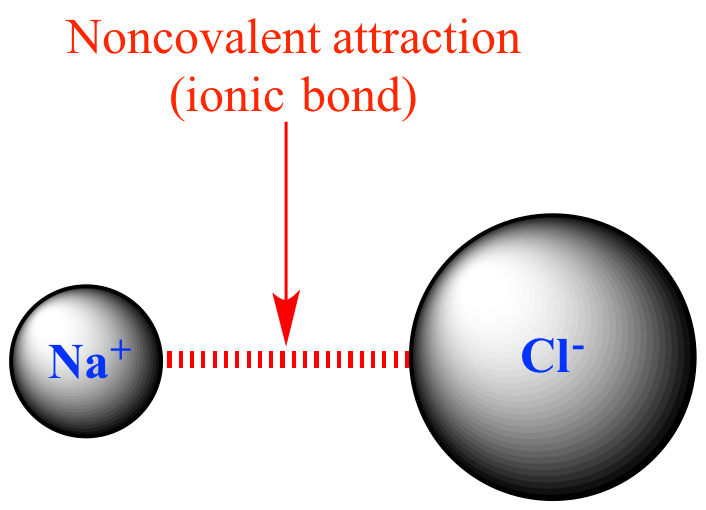
Illustrated Glossary of Organic Chemistry Anioncation interaction
All electromagnetic waves travel at the speed of light ( c ), or 2.998 × 108m / s. The relationship between the wavelength, frequency and speed of an electromagnetic wave is given by the equation: c = λ × ν. Electromagnetic radiation also occurs as discreet packets of energy (or quanta) called photons. The energy per photon (in Joules) is.

2 M of 100 mL Na2 SO4 is mixed with 3 M of 100 mL NaCl solution and 1 M of 200 mL CaCl2 solution
The compound CaCl2 is made up of which ions? The cation is: x x He ю . Ca2+ The anion is: x x He - 8. CI CI" Part 2 (2 points) How many calcium ions are in the compound? 1 How many chloride ions are in the compound? Separate the following balanced chemical equation into its total ionic equation.
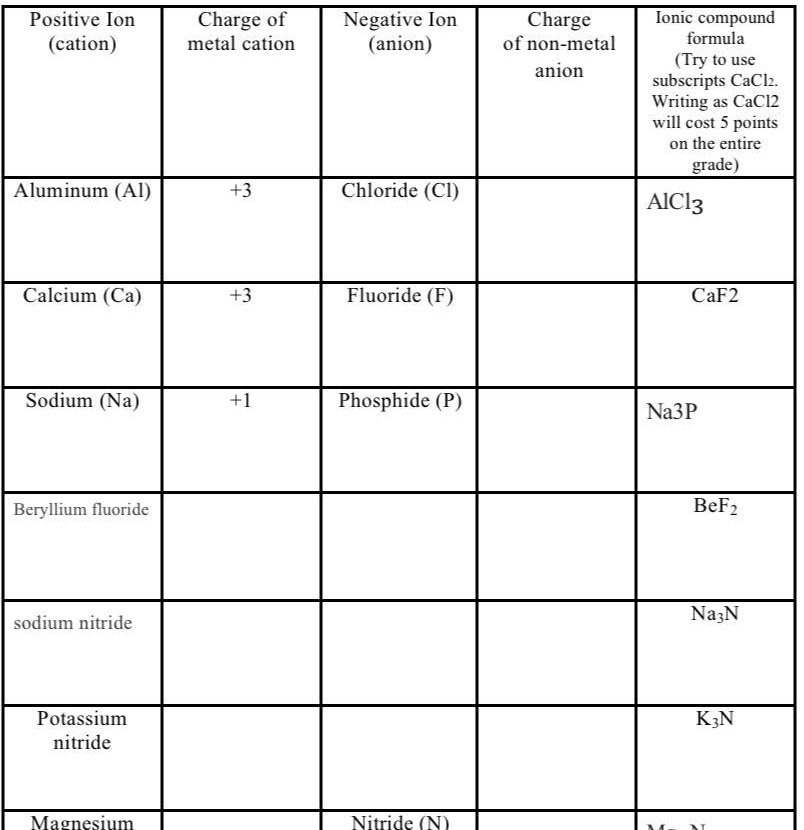
SOLVED 'I need help with this plz ??? Positive Ion (cation) Charge 0f metal cation Negative Ion
Chemical Compound Formulas Cacl2 Calcium Chloride - CaCl2 Table of Contents What is Calcium Chloride (CaCl 2 )? Properties of Calcium Chloride - (CaCl 2) Calcium Chloride structure (CaCl 2) Preparation of Calcium Chloride Calcium Chloride Solutions Calcium Chloride (CaCl 2) Uses Health Hazards Frequently Asked Questions

List of Cations and Anions Ion Hydrogen
The chemical formula of calcium chloride is: CaCl2. It is an ionic compound consisting of the calcium cation Ca2+ and two chlorine anions Cl−1. The bivalent calcium metal is forming an ionic bond with two chlorine atoms. Calcium chloride is an ionic compound. It is made of ions.

An ionic compound (salt) is composed of o... Physical Chemistry
CaCl2 is an ionic compound owing to the large electronegativity difference between the calcium atom and chlorine atom, which is greater than 2.0. In calcium chloride, the calcium atom donates its two electrons and become cation whereas each chlorine atom gain one electron, donated by Calcium, and get a negative charge.

SOLVED Possible Unknowns CaCO CaCl2 MgSO4 KC1 K2CO3 Na2CO3 NaC2HO2 NH4Cl (NH42SO4 Ca(NO2 KNO3
Adaptive Practice. Live Doubts. Video classes. All India test series. Live classes. Create your own tests. 24/7 Help. All Questions. About Us.

Cation vs AnionDifference between cation and anionCation and anion differenceCation and Anion
How to make the Chemical Formulas for a merging Cation and Anion? Basically, when a cation like Na (charge +1) and an anion like Cl (charge -1) combine, you get NaCl. . Same thing when Ca (charge +2) and O (charge -2) combine, you get CaO. But when. you combine Ca (charge +2) and Cl (charge -1) you get CaCl2, meaning two atoms of Cl.